
F gains 1 e - and Ogains 2 e -'s, and O gain e -'s to attain theconfiguration of Ne thus, the halogens tend to -1 charges and theoxygen family tends to a -2 charge. On the other side of the periodic table elements gain electrons toresemble the next higher noble gas. Thus, the group 2 metals tend tohave a +2 charge.

The alkali earth metals (group 2), such as, Mg or Sr lose two e-'sto attain the configuration of Ne. Since K loses one electron (1 negative charge)it is no longer neutral it now has a +1 charge. Kloses an electron so that it will have the same electronconfiguration as Ne.
#CHARGED PERIODIC TABLE FREE#
Another thing that makes electrons famous is their free movement around the. The negative charge of one electron balances the positive charge of one proton. As summarized in Table 2.1, protons are positively charged, neutrons are uncharged and electrons are negatively charged. The alkali metals will lose anelectron to resemble the next lowest noble gas thus, all the alkalimetals form +1 ions. + 1 for alkali metals, + 2 for for alkaline earth, + 3 for boron family, + / - 4 for carbon family, - 3 for nitrogen family, - 2 for oxygen family, - 1 for. Electrons are the subatomic particles characterized by their negative charges. All matter, including mineral crystals, is made up of atoms, and all atoms are made up of three main particles: protons, neutrons, and electrons.
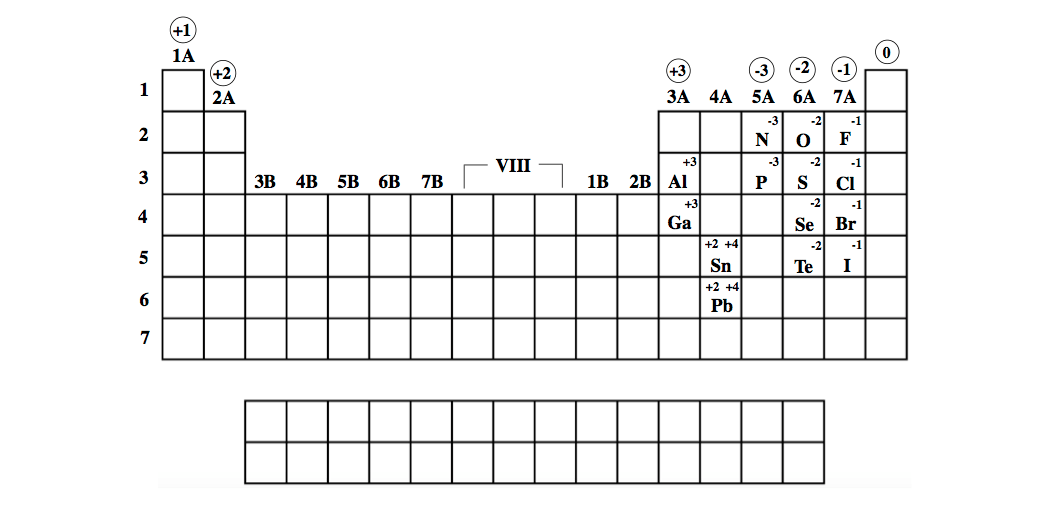
An element can attract additional electrons to achieve the electron configuration of the next highest noble gas.An element can give up electrons to achieve the electron configuration of the next lowest noble gas.Achieving a Noble Gas Electron Configurationįor reasons we will discuss later, elements react until theelement achieves an electron configuration of a noble gas.Įlements can achieve a noble gas electron configuration threeways.


 0 kommentar(er)
0 kommentar(er)
